Innovative Journey
Innovative Drugs
Innovative Drugs
We are dedicated to developing innovative drugs for cancer
and autoimmune diseases and ensuring our breakthroughs
to benefit as many patients as possible.
We believe in the value of continuous innovations and the
power of time. Our journey towards a healthier future starts
with molecules. We are continuously developing innovative
technologies and therapies while establishing a
comprehensive global IP system.
and autoimmune diseases and ensuring our breakthroughs
to benefit as many patients as possible.
We believe in the value of continuous innovations and the
power of time. Our journey towards a healthier future starts
with molecules. We are continuously developing innovative
technologies and therapies while establishing a
comprehensive global IP system.
Integrated Platform
Global Market
Global Market
We have built an integrated platform that spans from
innovation and clinical development to production and
commercialization, and have dedicated ourselves to
developing and delivering innovative therapies to patients
with cancer and autoimmune diseases worldwide.
innovation and clinical development to production and
commercialization, and have dedicated ourselves to
developing and delivering innovative therapies to patients
with cancer and autoimmune diseases worldwide.

InnoCare in Figures

2
Dual A+H Listing
First biotech listed on the HKEX in 2020
Fifth A+H listed biotech company
Fifth A+H listed biotech company
2
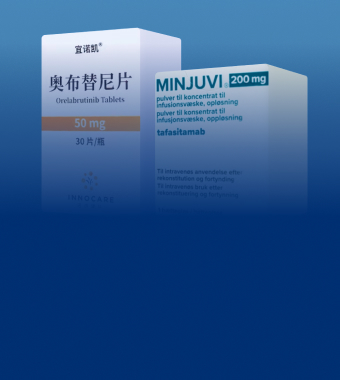
Marketed Products
Orelabrutinib approved for marketing in China
and Singapore, and included in China’s
National Reimbursement Drug List
Tafasitamab approved for marketing in Hong
Kong and approved for use in Bo‘ao and GBA
and Singapore, and included in China’s
National Reimbursement Drug List
Tafasitamab approved for marketing in Hong
Kong and approved for use in Bo‘ao and GBA

13
Clinical Pipelines
Global development
with strong capabilities
with strong capabilities
30
+
Clinical Trials
Clinical research in multiple locations
worldwide
worldwide

40%
+
R&D Personnel
Unlocking global talent and innovation
potential
potential

350
+
Patents and Patent
Applications
Applications
Continuously innovating to bring hope to
patients
patients










