Building a Leading Franchise in
Hemato-oncology
Hemato-oncology
Excellent Clinical Data
R/R CLL/SLL
0
1
2
3
4
5
6
7
8
9
0
1
2
3
4
5
6
7
8
9
.
0
1
2
3
4
5
6
7
8
9
%
Overall response rate (ORR)
0
1
2
3
4
5
6
7
8
9
0
1
2
3
4
5
6
7
8
9
%
Complete response (CR)
R/R MCL
0
1
2
3
4
5
6
7
8
9
0
1
2
3
4
5
6
7
8
9
%
Overall response rate (ORR)
0
1
2
3
4
5
6
7
8
9
0
1
2
3
4
5
6
7
8
9
.
0
1
2
3
4
5
6
7
8
9
months Median PFS
R/R MZL
0
1
2
3
4
5
6
7
8
9
0
1
2
3
4
5
6
7
8
9
.
0
1
2
3
4
5
6
7
8
9
%
Overall response rate (ORR)
0
1
2
3
4
5
6
7
8
9
0
1
2
3
4
5
6
7
8
9
%
Estimated 12-month overall survival (OS)
Huge Potential in
Autoimmune Diseases
Autoimmune Diseases
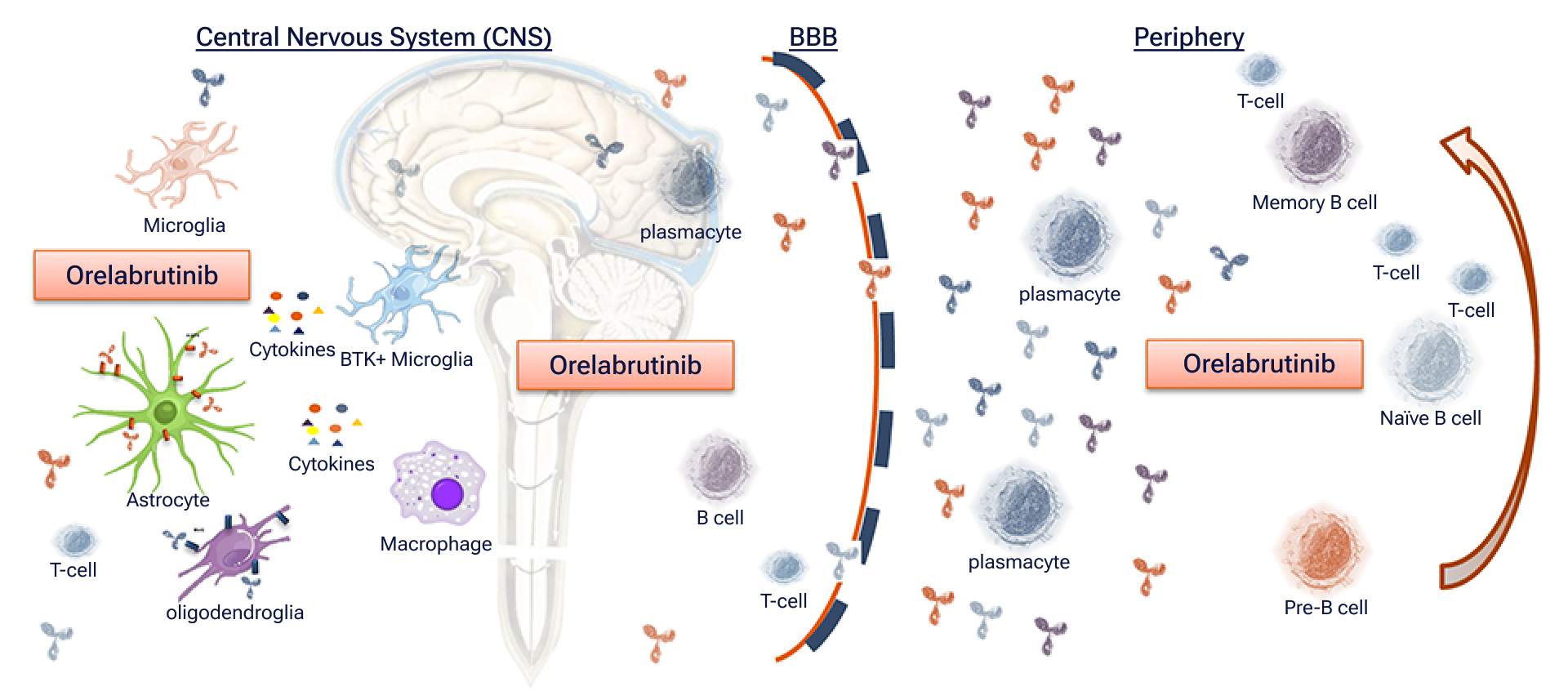
Blood-Brain Barrier Penetration
There are no BTK inhibitors approved for marketing for the treatment of autoimmune disease worldwide. However, orelabrutinib has the potential to be effective in the treatment of demyelinating diseases in the central nervous system and periphery. Its high target selectivity, good safety profile and blood-brain barrier (BBB) penetration capability provide a promising treatment option for autoimmune diseases.

Immune Thrombocytopenic Purpura (ITP)
The Phase III registrational trial of orelabrutinib for the treatment of ITP is expected to complete patient enrollment by the end of 2024.
According to Phase II study results, both 50mg QD and 30mg QD of orelabrutinib were safe in the treatment of patients with ITP. 40% at the 50mg arm reached the primary endpoint.
Among patients with primary endpoint response, 83.3% achieved adurable response. Among the patients with previous responses to glucocorticoid (GC) or intravenous immunoglobulin (IVIG), 75.0% at the 50mg arm met the primary endpoint.
Multiple Sclerosis (MS)
The 24-week data of orelabrutinib from the multiple sclerosis (MS) global Phase II trial is consistent with the previous reported 12-week data in terms of both efficacy and safety. The primary endpoint was achieved dose-dependently (Cmax driven) in all three active orelabrutinib treatment groups. The 80 mg QD cohort showed the highest reduction rate of cumulative number of new lesions Gd+T1 lesions and the best for lesion control throughout 24 weeks with best safety profile, indicating its potential as a leading MS treatment therapy.
| Cumulative number of New Gd+ T1 Lesion from Week 4 to Week 24 | Placebo / Orela 50mg QD (N=27) | Orela 50mg QD (N=30) | Orela 50mg BID (N=29) | Orela 80mg QD (N=29) |
| Adjusted mean cumulative number (95% CI) of lesions from W4 to W24 | 6.45 (3.62, 11.52) | 2.10 (0.62, 7.11) | 1.08 (0.30, 3.81) | 0.50 (0.09, 2.74) |
| Percent reduction | 67.4 (-22.0, 91.3) | 83.3 (33.2, 95.8) | 92.3 (56.5, 98.6) | |
| P-value | 0.0958 | 0.0114 | 0.0037 |
Systemic Lupus Erythematosus (SLE)
The Phase IIa trial for SLE demonstrated positive results, with remarkable SLE Responder Index (SRI)-4 response rates observed in a dose dependent manner, showing an improved dose-dependent efficacy. The Company expects to complete patient enrollment in 2024. We hope that orelabrutinib will become a first-in-class BTK inhibitor and serve as a potential treatment option for SLE.
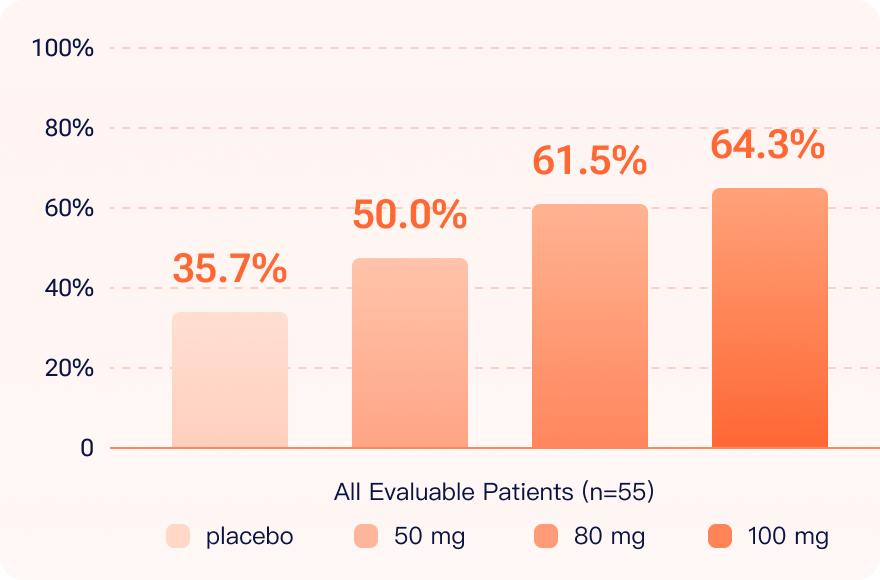
SLE Responder Index (“SRI”)-4 response rates
increased in a dose dependent manner
increased in a dose dependent manner