Tafasitamab Mechanism of Action
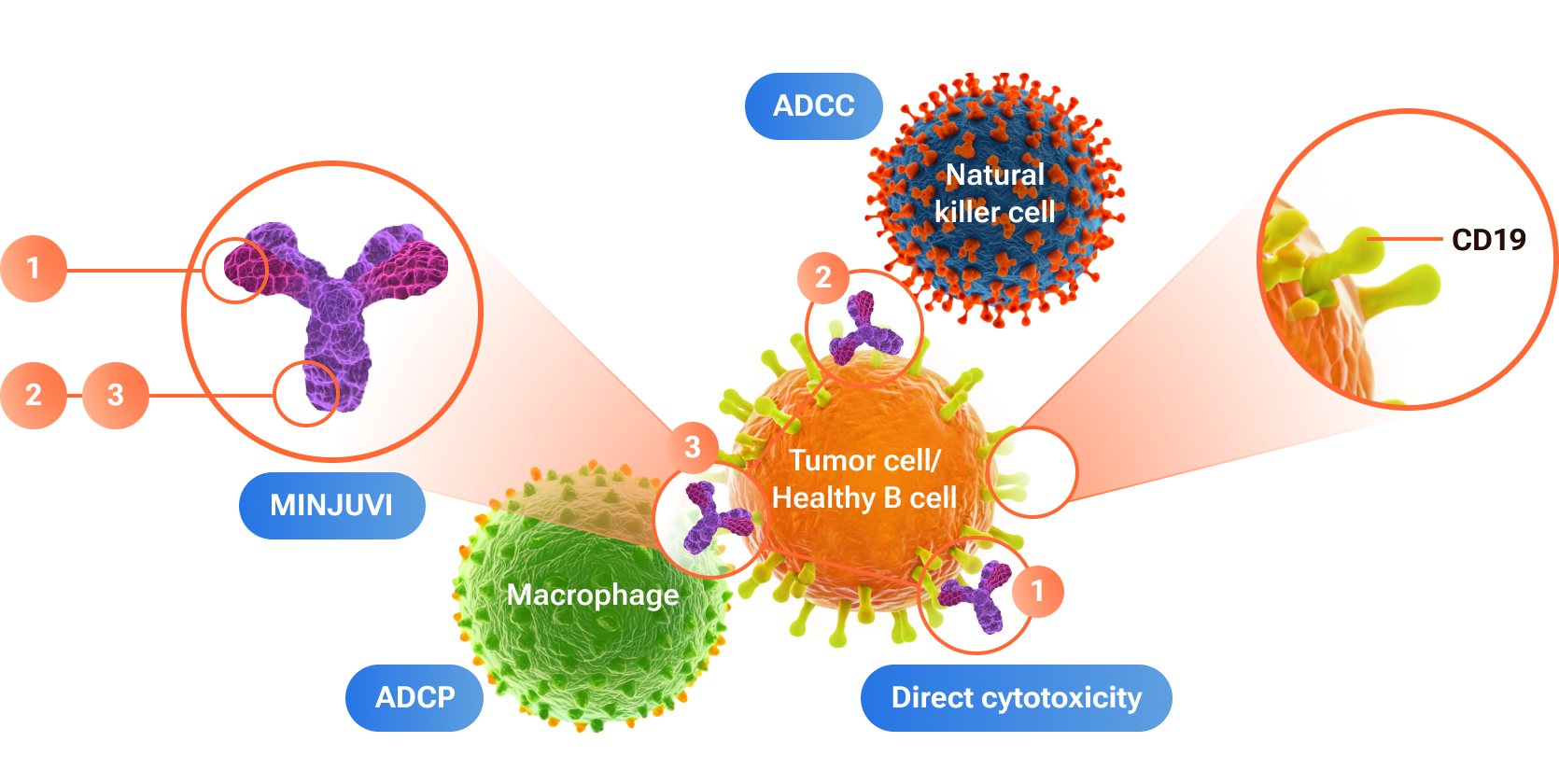
ADCC:Antibody-dependent cell-mediated cytotoxicity
ADCP:Antibody-dependent cellular phagocytosis
Clinical Data
The result of a five-year study from L-MIND shows that tafasitamab provided prolonged, durable responses in patients
with relapsed or refractory DLBCL.
with relapsed or refractory DLBCL.
57.5% ORR
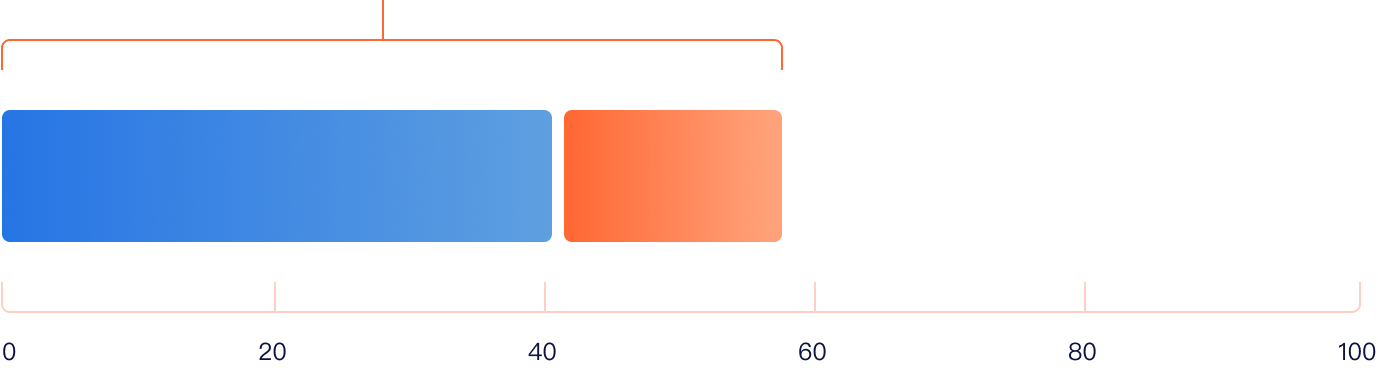
The ORR was 57.5%, with CR of 41.3% and PR of 16.2%.
41.3% CR
16.2% PR
Additional results include:
• Median duration of response (DoR) was not reached after a median follow up of 44.0 months.
• The median overall survival was 33.5 months and median progression-free survival was 11.6 months.
• Patients with one pLoT (n = 40) had a higher ORR of 67.5% (CR = 52.5% and PR = 15%).
• Median duration of response (DoR) was not reached after a median follow up of 44.0 months.
• The median overall survival was 33.5 months and median progression-free survival was 11.6 months.
• Patients with one pLoT (n = 40) had a higher ORR of 67.5% (CR = 52.5% and PR = 15%).
Benefits for Patients
Tafasitamab has been approved for marketing in Hong Kong and approval for use in Bo’ao and the Greater Bay Area. In Mainland China, patient enrollment of the registrational trial of tafasitamab in combination with lenalidomide was completed in China. The Company expects to submit the biologics license application (BLA) in the second quarter of 2024.
Thanks to the early access program in Bo’ao and the Greater Bay Area, the prescriptions of tafasitamab in combination with lenalidomide was filed at the Ruijin Hainan Hospital and Guangdong Clifford Hospital, bringing hope to DLBCL patients in China.